The Institute of Solid State Physics (ISSP) at the Hefei Institutes of Physical Science — one of the major research institutes within the Chinese Academy of Sciences (CAS) — develops advanced materials, photonics, and functional devices that bridge fundamental solid-state science and practical applications. A recent high-profile outcome from ISSP is the work led by Professor Jiang Changlong and his research team: a wearable fluorescent eye-patch sensor that noninvasively detects lysozyme in human tears — a biomarker linked to ocular surface health and certain eye diseases.
he sensor was developed by Prof. Jiang Changlong’s laboratory at the Institute of Solid State Physics, Hefei Institutes of Physical Science, CAS. The technology and validation studies were presented in peer-reviewed form (published in Analytical Chemistry) and publicized by the Hefei Institutes and CAS news outlets. The paper’s experimental authors (lead author Lei Pan and collaborators) describe the design, synthesis, and performance of a Eu–Dy metal-organic-framework (MOF) hydrogel used as the sensing element.
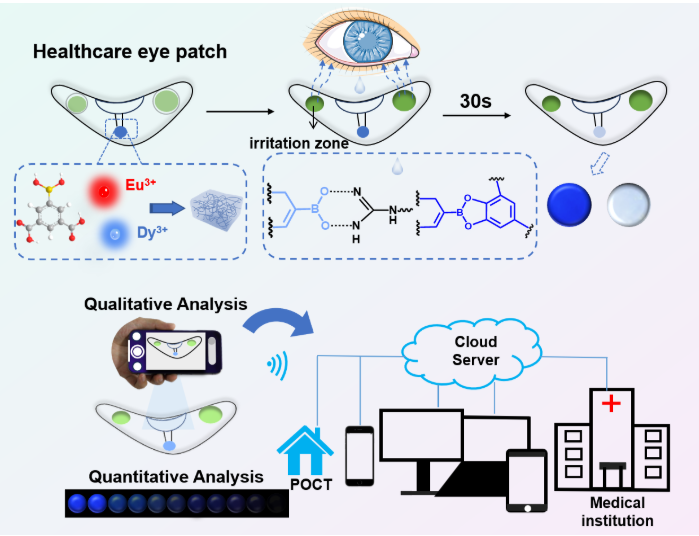
Rather than an electronic circuit or continuous electronic readout, this device is a soft, biocompatible patch placed under the lower eyelid (on the skin just below the eye) that passively collects tear fluid. The sensing element is a multi-emission MOF hydrogel doped with rare-earth ions (europium, Eu³⁺, and dysprosium, Dy³⁺). Under UV excitation the MOF hydrogel fluoresces; interaction of lysozyme molecules with the MOF matrix produces measurable changes in the fluorescence signal that can be photographed and quantified using a smartphone camera and image-processing software. The system therefore provides a noninvasive, wearable, and smartphone-compatible route to monitor a tear fluid biomarker linked to eye disease risk.
Sensing chemistry & materials
- Sensing element: A metal-organic framework (MOF) hydrogel synthesized by coordinating Eu³⁺ and Dy³⁺ ions with an organic ligand (reported ligand: 5-boronobenzene-1,3-dicarboxylic acid in the published protocol). The MOF forms a gel network compatible with soft, skin-contact formats.
- Rare-earth photophysics: The organic ligand acts as an “antenna” that absorbs excitation energy and transfers it to lanthanide centers; Eu and Dy provide distinct emission features and robust luminescence (the team exploited multi-emission behavior to enable sensitive visual readout).
Optical excitation & readout
- Excitation: The MOF gel is excited in the near-UV (the published work used ~275 nm excitation in controlled tests), producing bright fluorescence visible under UV illumination.
- Detection method: The fluorescent patch is imaged (photographed) under UV illumination using an ordinary smartphone camera. Image analysis (color/brightness ratios) quantifies the fluorescence change caused by lysozyme binding/quenching. This design emphasizes low-cost, portable readout without specialized spectrometers.
Analytical performance
- Analyte: Lysozyme, a tear-film protein implicated in ocular defense and linked to inflammatory and infectious conditions when abnormal.
- Sensitivity: The reported detection limit is very low — on the order of ~1.5 nanomolar — demonstrating high analytical sensitivity suitable for physiological tear concentrations.
- Selectivity & mechanism: The MOF hydrogel’s fluorescence is attenuated (quenched) when lysozyme interacts with the framework; the team characterized the response and established calibration curves to relate fluorescence change to lysozyme concentration.
Wearable format & user interaction
- Form factor: Soft hydrogel patch, thin and conformal, designed to sit beneath the lower eyelid margin where it receives tear fluid via capillary action.
- User flow: Apply patch → wait short collection period → illuminate with small UV lamp (or integrated UV LED) → photograph with smartphone → smartphone app interprets color/fluorescence to report lysozyme level and, if desired, flags abnormal values for follow-up.
Clinical & practical implications
- Early warning: Lysozyme fluctuation may indicate ocular surface inflammation, infection risk, or tear-film dysfunction; an easy, wearable sensor could enable home monitoring and early clinical referral.
- Point-of-care & telemedicine: Because readout uses a smartphone, the device is well suited for remote monitoring and tele-ophthalmology programs — data could be sent to clinicians for trend analysis.
- Low-cost scalability: MOF hydrogels and smartphone imaging lower instrument costs compared with laboratory assays, improving accessibility in community or resource-limited settings.
Chinese Academy of Sciences (CAS) team successfully demonstrates a proof-of-concept for a wearable MOF hydrogel eye patch that can sensitively, accurately, and noninvasively measure lysozyme in tears, with readout accessible by smartphone imaging. It contributes to the field of wearable diagnostics and ocular health monitoring by combining advanced materials (Eu-Dy MOF), fluorescence sensing, and practical device integration.
Metrics & Performance Summary
| Metric | Value / Range |
|---|---|
| Limit of Detection (LOD) | ~1.5 nM lysozyme |
| Recovery in tear fluid | ~94-110% |
| Fluorescence mechanism | Blue fluorescence under UV, quenched by lysozyme (static quenching + electron transfer) |
| Wearable format | Eye patch under lower eyelid, read by smartphone image under UV light |
To move toward eventual real-world use, the typical next steps include more extensive in vivo trials, optimizing safety (especially UV exposure), refining app calibration, improving durability, and ensuring comfortable wear over longer time spans.
Materials & Methods
Sensor Material
- The sensing element is a hydrogel patch functionalized with a metal-organic framework (MOF) incorporating rare earth ions Eu³⁺ (europium) and Dy³⁺ (dysprosium).
- The organic ligand used is 5-boronobenzene-1,3-dicarboxylic acid which coordinates with Eu³⁺ and Dy³⁺ to form the MOF gel network.
Optical Properties / Fluorescence
- The MOF gel exhibits multi-emission fluorescence upon excitation with ~275 nm ultraviolet light. The organic ligand absorbs light (antenna effect) and transfers energy to the rare earth centers; Eu and Dy produce visible emission. The emission includes a blue fluorescence component that is significant in the readout.
Detection Mechanism
- Quenching: Lysozyme interacts with the MOF gel by forming complexes, and through local electron transfer and static quenching effects reduces certain fluorescence emissions (notably the blue component).
- The reduction of fluorescence is correlated with lysozyme concentration.
Device / Wearable Format
- The MOF gel is incorporated into an eye patch, placed under the lower eyelid (skin under eye) where it can contact tear fluid.
- After a specified exposure / collection time, the patch is illuminated (UV), photographed by a smartphone, and the image is analyzed via a color recognition app to extract quantitative fluorescence intensity or color ratio metrics.
Results
- Detection limit (LOD): ~ 1.5 nanomolar lysozyme. Thus quite sensitive compared to many existing methods.
- Recovery in tear fluid samples: Ranges from 94% to 110%, meaning that when known lysozyme is spiked into actual tear fluid, the sensor can recover nearly all or slight overestimates—i.e. good accuracy.
- The fluorescence color visibly changes in the patch under UV with varying lysozyme concentrations; images show that increasing lysozyme leads to more quenching (darker/less fluorescence), especially in the blue part. These visible changes are sufficient to be captured by phone for quantification.
Smart Eye Patch Uses Fluorescence to Monitor Eye Health—-Chinese Academy of Sciences
