BioIntelliSense’s BioButton® is an FDA-cleared wearable medical device designed for continuous, multi-parameter patient monitoring. It facilitates seamless tracking of vital signs and physiological biometrics, enhancing patient care both in hospital settings and at home.

In-Hospital Monitoring: The BioButton® is utilized across medical-surgical units, specialty care areas, and emergency departments, automating vital sign collection and enhancing patient care efficiency.
Remote Patient Monitoring: Its design supports hospital-level care at home, facilitating continuous monitoring for patients transitioning from hospital to home care.
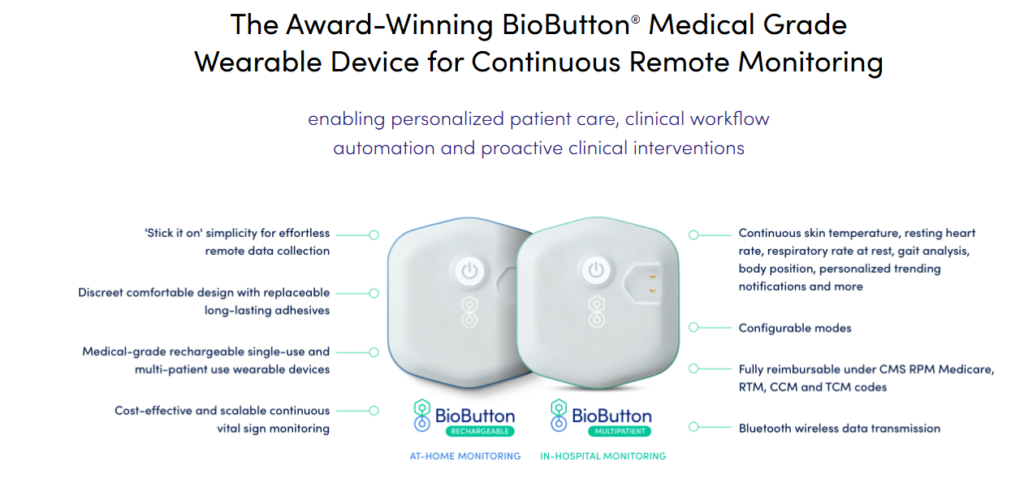
Virtual Care Programs: The device’s scalability and interoperability make it ideal for expanding hospital virtual care programs, providing cost-effective solutions for continuous patient monitoring.
Medical grade data capture and insights with clinical accuracy physicians can trust
Data analytics and services to enable early detection of adverse vital sign trends
Cost-effective continuous physiologic monitoring with the award-winning BioButton single-use and multi-patient use wearable medical devices
