A zinc-air battery is a type of metal-air battery powered by the oxidation of zinc and reduction of oxygen from the air. These batteries are known for their high energy density, low cost, and eco-friendly components, making them ideal for applications ranging from hearing aids to grid-scale energy storage.
However, conventional zinc-air batteries have been limited by issues like poor rechargeability, sluggish oxygen reaction kinetics, and the need for expensive catalysts.
A typical dual-atom zinc-air battery consists of:
- Anode: Zinc metal, which is oxidized during discharge.
- Cathode: Air electrode embedded with a dual-atom catalyst to facilitate oxygen reactions.
- Electrolyte: Usually an alkaline medium such as potassium hydroxide (KOH) or solid-state electrolyte for enhanced safety.
During discharge, oxygen from the air reacts with hydroxide ions to form water, while zinc is oxidized to zincate or zinc oxide. During charging, this process reverses with the help of the catalyst.
The dual-atom zinc-air battery represents a significant leap forward in battery technology. By merging cutting-edge catalysis with sustainable design, it offers a glimpse into a future where energy is clean, affordable, and accessible. As innovation continues to push boundaries, dual-atom batteries may soon become the backbone of next-generation energy storage systems.
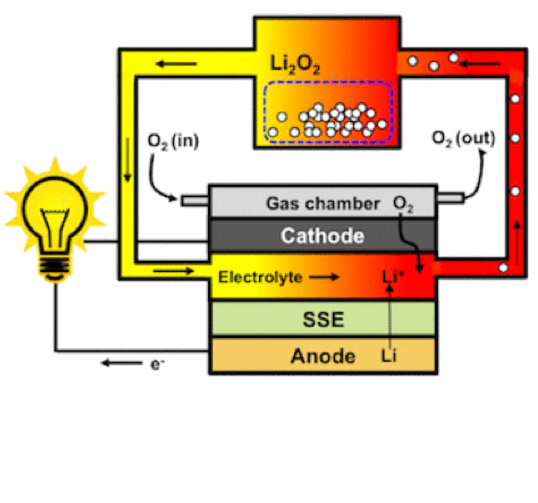
Zinc-air batteries harness the oxidation of zinc with oxygen from the air to generate electricity. These batteries typically consist of a zinc anode, an air cathode, and an electrolyte. The oxygen reduction reaction (ORR) at the cathode is a crucial step in this process, but it often suffers from slow kinetics, which limits the battery’s performance.
Dual-Atom Catalysts
The game-changer in the latest research is the introduction of dual-atom catalysts. In this approach, two types of metal atoms (often a transition metal like cobalt, iron, or nickel paired with zinc) are precisely anchored on a nitrogen-doped carbon substrate. This combination creates synergistic catalytic sites that enhance both the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER), the two key electrochemical processes in a rechargeable zinc-air battery.
Why Dual Atoms Matter
- Enhanced Catalytic Activity: Dual-atom sites offer more efficient and stable pathways for oxygen reactions than single-atom or nanoparticle-based catalysts.
- Better Charge-Discharge Efficiency: The improved oxygen kinetics reduce overpotentials during charging and discharging.
- Longer Cycle Life: Batteries with dual-atom catalysts tend to maintain performance over hundreds or even thousands of cycles.
- Reduced Cost: These systems often avoid using precious metals like platinum or iridium, which reduces cost and increases scalability.
The dual-atom zinc-air battery holds promise in several sectors:
- Grid-Scale Storage: With abundant zinc and ambient air as resources, these batteries are ideal for storing renewable energy from solar and wind.
- Electric Vehicles (EVs): Their lightweight and high energy density make them a strong contender for next-generation EV batteries.
- Consumer Electronics: Enhanced rechargeability and longevity could power portable devices more efficiently.
- Remote and Off-Grid Areas: The use of inexpensive and non-toxic materials makes them ideal for decentralized energy systems.
To overcome the limitations of ORR, researchers have been exploring various catalysts. Platinum-based catalysts are traditionally used, but their high cost and susceptibility to impurities make them less than ideal. A research team at Tohoku University’s Advanced Institute of Materials Research (WPI-AIMR), led by Assistant Professor Di Zhang, has made a significant breakthrough by developing a dual-atom catalyst made of iron (Fe) and cobalt (Co), combined with nitrogen (N) and carbon (C) in a porous structure. This catalyst, named Fe1Co1-N-C, has demonstrated remarkable efficiency in facilitating the oxygen reduction reaction in alkaline conditions.
Key Features of the New Catalyst
- Dual-Atom Design: The catalyst’s unique design, featuring two metal atoms closely paired together, enhances catalytic activity.
- Material Composition: The combination of iron, cobalt, nitrogen, and carbon creates an optimal structure for efficient oxygen reduction.
- Porous Structure: The catalyst’s porous structure, achieved through a method involving hard templates and a CO2 activation process, allows reactants to move through the material easily, improving overall catalytic performance.
How It Was Developed
The Fe1Co1-N-C catalyst was designed using a combination of computational modeling and experimental techniques. The researchers first used a model to predict how pH (acidity) affects the reaction, which guided them in creating a catalyst with the right properties for maximum efficiency.
The enhanced performance of the dual-atom catalyst can be attributed to its ability to catalyze the ORR more efficiently than traditional catalysts. This leads to improved battery performance, higher energy efficiency, and a longer cycle life. Moreover, dual-atom catalysts are often more stable and durable than single-atom catalysts, ensuring consistent performance over multiple charge-discharge cycles.
