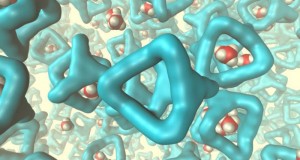
Researchers in the School of Chemistry and Chemical Engineering at Queen’s, along with colleagues at the University of Liverpool, UK, and other, international partners, have invented the new liquid and found that it can dissolve unusually large amounts of gas, which are absorbed into the ‘holes’ in the liquid. Porous materials, as their name would suggest, are materials with holes. These are materials that look totally solid to the naked eye, but on a molecular level, contain lots of empty space. Useful as both catalysts and molecular separators, porous solids have seen their way into a variety of industrial applications, including plastics and petrol manufacturing. The three-year research project could pave the way for many more efficient and greener chemical processes, including ultimately the procedure known as carbon capture trapping carbon dioxide from major sources, for example a fossil-fuel power plant, and storing it to prevent its entry into the atmosphere.
Professor Stuart James of Queen’s School of Chemistry and Chemical Engineering said: “Materials which contain permanent holes, or pores, are technologically important. They are used for manufacturing a range of products from plastic bottles to petrol. However, until recently, these porous materials have been solids. What we have done is to design a special liquid from the ‘bottom-up’. We designed the shapes of the molecules which make up the liquid so that the liquid could not fill up all the space. Because of the empty holes we then had in the liquid, we found that it was able to dissolve unusually large amounts of gas. These first experiments are what is needed to understand this new type of material, and the results point to interesting long-term applications which rely on dissolution of gases. “A few more years’ research will be needed, but if we can find applications for these porous liquids they could result in new or improved chemical processes. At the very least, we have managed to demonstrate a very new principle, that by creating holes in liquids we can dramatically increase the amount of gas they can dissolve. These remarkable properties suggest interesting applications in the long term.”
In the journal Nature, a team of chemical engineers describes the world’s first bonafide porous liquid, a solution consisting of organic “cage molecules” designed to enclose empty space. These molecular cages are dissolved in an organic solvent that provides fluidity, but whose molecules are too large to enter the cage. The liquid contains hundreds of times more empty space than traditional fluids, and it turns out to be great at soaking up methane, a potent greenhouse gas. The first porous liquid is specially designed of cage-like molecules sized to capture small molecules like methane or carbon dioxide, but too large to be filled by the liquid solvent in which they are dissolved. The resulting soup behaves quite differently from the standard liquid. For example, the porous liquid can capture up to 8 times more methane gas than could be dissolved.
Most carbon capture and storage systems depend on liquid solvents or reaction systems, because liquid systems more easily retrofit onto existing power plants. Current technology relies mostly on amines or carbonates to capture the carbon dioxide from the power plant emission stream. The carbon dioxide can then be released from the solvents and liquefied for injection into storage sites. Alternatives like engineered porous liquids could offer better options for carbon capture, a technology which may be essential to bridge the gap between increasing emissions of global warming gases and commitments to adopt alternative energy solutions to reduce these climate changing emissions. Even if we can avoid the risks of storing carbon dioxide deep in the earth, porous liquids could become important for other technological breakthroughs. We cannot help but think of most of the biochemical miracles our bodies perform; our cells use molecules built precisely to catalyze the chemical reactions we need to survive, all in the liquid medium of our watery beings.
For more information please visit: www.qub.ac.uk

Comments are closed.