Genetic-developmental theory states that individual differences in a continuous phenotype result from the action of a large number of genes, each exerting an effect that works with environmental factors to produce the trait.
This type of trait is influenced by multiple factors making it more complex and difficult to study than a simple Mendelian trait (one gene for one phenotype).
Aggression, as well as other behavioral traits, is studied genetically based on its heritability through generations. Heritability models of aggression are mainly based on animals due to the ethical concern in using humans for genetic study. Animals are first selectively bred and then placed in a variety of environmental conditions, allowing researchers to examine the differences of selection in the aggression of animals.
Both genes and environment contribute to individual differences in aggression. Surveys of the pathways implicated in the physiological and neuronal processes involved highlight the potential role of genes regulating sexual differentiation, anxiety, stress response and neurotransmission.
To date, however, association studies have provided little evidence of a substantially significant role for any single candidate gene in such pathways. This may be because genes function against a background in which other genetic and environmental factors are crucial. A series of recent studies, particularly concentrating on monoamine oxidase A, has emphasised the necessity of examining gene by environmental interactions if the contributions of individual loci are to be understood.
Aggression is a multi-dimensional concept, but it can be generally defined as behavior that inflicts pain or harm on another.
These findings have major significance for the interpretation of data, both from individual gene and whole genome association studies. Functional imaging studies of genetic variants affecting serotonin pathways have also provided valuable insights into potential links between genes, brain and aggressive behaviour.
Key Concepts:
- Aggression is an evolutionarily advantageous trait with input from one of the most primitive brain regions, the amygdala.
- There is significant disparity between aggressive behaviour in males and females.
- Genes and environment both influence aggressive behaviour and there is evidence that stressful life events can interact with specific genetic variants.
- DBH, COMT, adrenergic receptors, NET1 and SLC6A2 have been studied as possible candidate genes linking stress and aggression.
- In the serotonin system, genetic polymorphisms in MAOA, SLC6A4, TPH1/2 and the serotonin receptor genes have been linked with aggression.
- Studies have shown a potential link between diet and its effects (e.g. on glucose levels) and aggression.
- Brain imaging studies are beginning to assist an interpretation of the links between genetic variation and aggression
The heritability of aggression has been observed in many animal strains after noting that some strains of birds, dogs, fish, and mice seem to be more aggressive than other strains. Selective breeding has demonstrated that it is possible to select for genes that lead to more aggressive behavior in animals.[7] Selective breeding examples also allow researchers to understand the importance of developmental timing for genetic influences on aggressive behavior.
A study done in 1983 (Cairns) produced both highly aggressive male and female strains of mice dependent on certain developmental periods to have this more aggressive behavior expressed. These mice were not observed to be more aggressive during the early and later stages of their lives, but during certain periods of time (in their middle-age period) were more violent and aggressive in their attacks on other mice.[8] Selective breeding is a quick way to select for specific traits and see those selected traits within a few generations of breeding. These characteristics make selective breeding an important tool in the study of genetics and aggressive behavior.
Jacson Lab report :
The prevalence of violence in our society has motivated biomedical researchers, sociologists and psychologists to look for genetic markers, predictors and causes for this destructive human behavior. Advances in neurochemistry and imaging technology have shown that many emotional and control disorders such as violence, suicide, depression and anxiety, involve disruptions in the brain’s normal activity due to altered gene expression, chemical imbalances, and environmental factors.
Violent genes
In developed countries, the majority of all violent crime is committed by a small group of antisocial repeat offenders. But until recently, no genes had been shown to contribute to severe or recidivistic violent behaviors such as homicide. According to a meta-analysis on data from 24 genetically informative studies, up to 50% of the total variance in aggressive behavior is explained by genetic influences.
Nature and nurture
Both our genotype and the environmental factors to which we are exposed to throughout life contribute to shaping our brain functions. Changes in the expression of specific genes in the brain -such as MAOA, DAT1 and DRS2– can affect neurotransmitter levels, which, in turn, influences complex functions such as intelligence, mood and memory. Environmental influences including stress, substance abuse, diet, sleep quality and social relationships also affect the brain.
The warrior gene
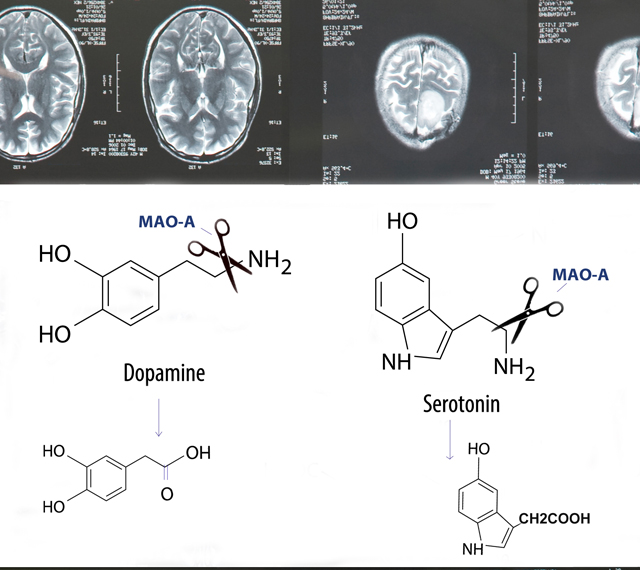
Monoamine oxidase A (MAO-A) is an enzyme that normally functions in neuronal mitochondria by breaking down several key neurotransmitters: serotonin, dopamine and norepinephrine which are important in aggression, emotion and cognition (Figure 1). The MAOA gene –located in the X chromosome- is also known as the warrior gene, since abnormal versions of the gene often result in aggressive behaviors.
Several animal models in which the function of MAO-A is defective display excessive levels of serotonin, dopamine, and norepinephrine (noradrenaline) in the brain. Additionally, MAO-A mutant mice have increased reactivity to stress and increased aggression to intruder mice compared to normal mice. Humans carry different variants of the MAOA gene that result in different levels of gene expression. “Normal” individuals carry MAO variants that express high levels of neuronal MAO-A, a small subset of patients carry MAO variants that result in the absence of functional MAO-A, while other people carry variants that result in low levels of MAO-A expression in the brain.
Interestingly, MAO-A’s function affects the following systems:
- The dopamine system, which is involved in mood, motivation and reward, arousal, and other behaviors.
- The serotonin system, which is involved in impulse control, affect regulation, sleep, and appetite.
- The epinephrine/norepinephrine system, which facilitates fight-or-flight reactions and autonomic nervous system activity.
In the early 1990s, researchers linked low levels of MAO-A with increased frequencies of antisocial behavior, specifically when individuals had a history of being mistreated during childhood. Later studies by Guo and colleagues investigated MAOA variants in 2500 American boys in grades 7 to 12, and demonstrated a genetic basis for severe aggressive behavior seen at school. A specific variant of the MAOA gene (VNTR 2R MAOA) was a risk factor of violent delinquency, but only when the boys suffered some other stress, such as family issues, low popularity and failing school.
These and other studies suggest that when subjected to an abusive childhood, individuals with low -MAO-A expression has are at an increased risk of developing Anti-Social Personality Disorder. This may yield to a long-term pattern of manipulating, exploiting, or violating the rights of others, and may commit violent criminal acts.
DAT1 gene
Many other studies on genetic variants and aggression have focused on the role of dopamine and its receptors and transport sites. The dopamine transporter (DAT1), which is encoded by the SLC6A3 gene, mediates the active reuptake and inactivation of dopamine from the synapse and is a principal regulator of dopaminergic neurotransmission. In homozygous DAT1-null mice, dopamine persisted at least 100 times longer in the extracellular space, providing a biochemical explanation of the hyperlocomotion (hyperdopaminergic phenotype) demonstrating the critical role of DAT1 in regulating neurotransmission.
Guo and collaborators analyzed a set of children (2500, seventh through twelfth graders) looking for additional genetic markers of aggression and demonstrated that a specific variant of the dopamine transporter 1 (DAT1) gene – the DAT1*10R genotype- contributed to serious delinquent behavior, compared to subjects homozygous for the DAT1*9R/9R allele. DAT1 normally limits the level and duration of dopamine receptor activation, thus controlling synaptic dopamine levels.
DRD2 gene
The D2 dopamine receptor is a G protein-coupled receptor is located on postsynaptic dopaminergic neurons and is centrally involved in reward-mediating mesocorticolimbic pathways. This receptor is coded by the DRD2 gene, that is involved in governs physiologic functions related to locomotion, hormone production, and drug abuse. Guo and colleagues also found that a DRD2 variant was a risk factor of violent delinquency, but only when adolescents and young adults suffered some other stress, such as family issues, low popularity and failing school.
Aggressive behavior in humans has also been linked with other genes, including variants of the androgen receptor gene (AR) and the catechol-O-methyltransferase (COMPT) gene –also responsible of breaking down dopamine.
https://www.jax.org/
