Injuries to peripheral nerves –– tissues that transmit bioelectrical signals from the brain to the rest of the body –– often result in chronic pain, neurologic disorders, paralysis or disability. Now, researchers have developed a stretchable conductive hydrogel that could someday be used to repair these types of nerves when there’s damage. They report their results in ACS Nano.
Injuries in which a peripheral nerve has been completely severed, such as a deep cut from an accident, are difficult to treat. A common strategy, called autologous nerve transplantation, involves removing a section of peripheral nerve from elsewhere in the body and sewing it onto the ends of the severed one. However, the surgery does not always restore function, and multiple follow-up surgeries are sometimes needed. Artificial nerve grafts, in combination with supporting cells, have also been used, but it often takes a long time for nerves to fully recover. Qun-Dong Shen, Chang-Chun Wang, Ze-Zhang Zhu and colleagues wanted to develop an effective, fast-acting treatment that could replace autologous nerve transplantation. For this purpose, they decided to explore conducting hydrogels –– water-swollen, biocompatible polymers that can transmit bioelectrical signals.
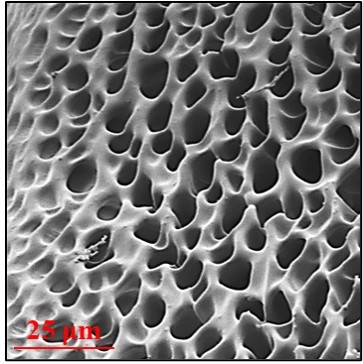
The researchers prepared a tough but stretchable conductive hydrogel containing polyaniline and polyacrylamide. The crosslinked polymer had a 3D microporous network that, once implanted, allowed nerve cells to enter and adhere, helping restore lost tissue. The team showed that the material could conduct bioelectrical signals through a damaged sciatic nerve removed from a toad. Then, they implanted the hydrogel into rats with sciatic nerve injuries. Two weeks later, the rats’ nerves recovered their bioelectrical properties, and their walking improved compared with untreated rats. Because the electricity-conducting properties of the material improve with irradiation by near-infrared light, which can penetrate tissues, it could be possible to further enhance nerve conduction and recovery in this way, the researchers say.
The authors acknowledge funding from the National Key Research and Development Program of China, the National Natural Science Foundation of China, the Program for Changjiang Scholars and Innovative Research Team in University, and Program B for Outstanding Ph.D. Candidate of Nanjing University.
NIH –
Peripheral nerve injury (PNI) causes severe motor disability, sensory aberrations, and pain syndromes in patients, seriously restricting social development . Autologous nerve grafting is currently regarded as the gold standard for reconstruction of nerve damage in the clinic. However, autografts are limited by considerable disadvantages, including poor functional recovery, finite availability, donor site function loss, morbidity, and pain 5. Nerve guidance conduits (NGCs) have emerged as a potential alternative to autologous nerve grafts due to their ability to reduce neuroma formation and prevent axonal escape. Moreover, the repair of small defects is achievable using clinically available NGCs . However, effective and efficient functional reconstruction of larger and complex defects remains clinically challenging . Therefore, providing an effective method to bridge the gap in nerve regeneration represents a focus of research into peripheral nerve regeneration, especially the construction of a beneficial intraluminal microenvironment of NGCs.
In the pursuit of an effective nerve conduit, various physical and biological strategies have been employed. Progress in research associated with elucidating the intrinsic mechanism of peripheral nerve repair has increased the focus on the design of scaffold materials that simulate the repair process such as constructing the neurovascular microenvironment. Unlike axons in the central nervous system (CNS), injured peripheral nerves exhibit the capacity to regenerate largely due to the supportive population of Schwann cells (SCs), which are primary glial cells in the peripheral nervous system (PNS). SCs have a unique capacity to de-differentiate, re-enter the cell cycle, and subsequently myelinate regenerating axons, which can promote regeneration by secreting neurotrophic molecules and establishing a supportive growth matrix. SCs play an important role in peripheral nerve repair not only in the early clearance of debris and control of inflammation but also in axon growth and myelination
courtesy : NIH.gov & ACS.org
