EyeArt is the first FDA cleared AI technology for autonomous detection of both more than mild and vision-threatening diabetic retinopathy. It is the most extensively validated autonomous AI technology, tested in the real-world on more than half million patients and nearly two million retinal images globally..
EyeArt is the most extensively validated AI technology in the world. It has been tested in a clinical validation study on over 100,000 patient visits, one of the largest data sets used to test any available DR screening technology, in demanding, real-world clinical environments using images captured in everyday practice. It has also been independently validated by UK NHS in a study with over 20,000 patients.
Fully automated DR screening, including imaging, grading for DR in accordance with internationally recognized standards and reporting, in a single office visit. No specialist needed for DR screening. EyeArt also flags poor quality images or images not showing the required retinal fields (protocol deviations) automatically.
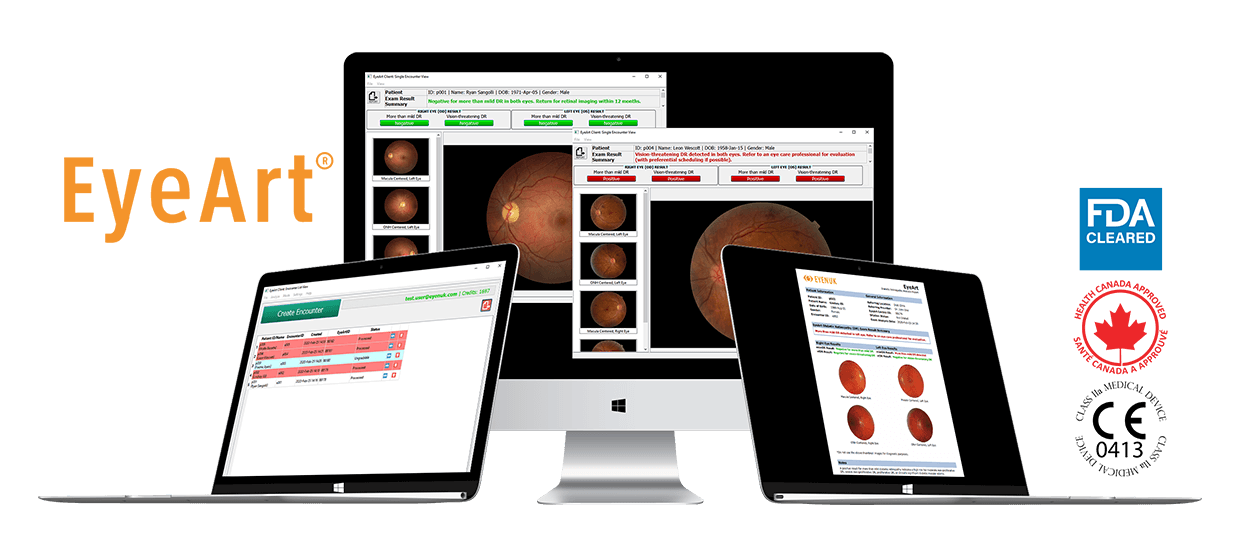
Once the patient fundus images have been captured and submitted to EyeArt, the DR screening results are available to view and export to a PDF report in less than a minute.
The high-performance EyeArt AI algorithm automatically detects diabetic retinopathy quickly, accurately, and consistently, with higher sensitivity than an eye care expert trained in image grading.
The EyeArt system is easy to use and with minimal training can be employed by nurses or technicians, enabling diabetic retinopathy screening at the point of care and eliminating an eye care specialist appointment just for screening.
EyeArt makes in-clinic, real-time diabetic retinopathy (DR) screening possible for primary care practices, diabetes centers and optometric offices by allowing physicians to quickly and accurately identify referable DR patients during a diabetic patient’s regular exam.
In a study with over 100,000 consecutive patient visits, EyeArt reports a 91.3% sensitivity and 91.1% specificity for referable DR and a 98.5% sensitivity for vision-threatening DR.
The technology is designed to work effectively with image quality commonly encountered in diabetes patients and with imaging protocols/cameras typically used in screening setups. EyeArt is HIPAA compliant and keeps all data secure and private.

STEP 1
Capture color retinal fundus
images of the patient’s eyes

STEP 2
Submit images to the
cloud for analysis

STEP 3
Download DR screening results and
export PDF report.
Any physician can quickly and accurately detect more than mild and vision-threatening diabetic retinopathy in patients during a diabetic patient’s regular exam with a report that is generated in less than 60 seconds after submission of patient images.
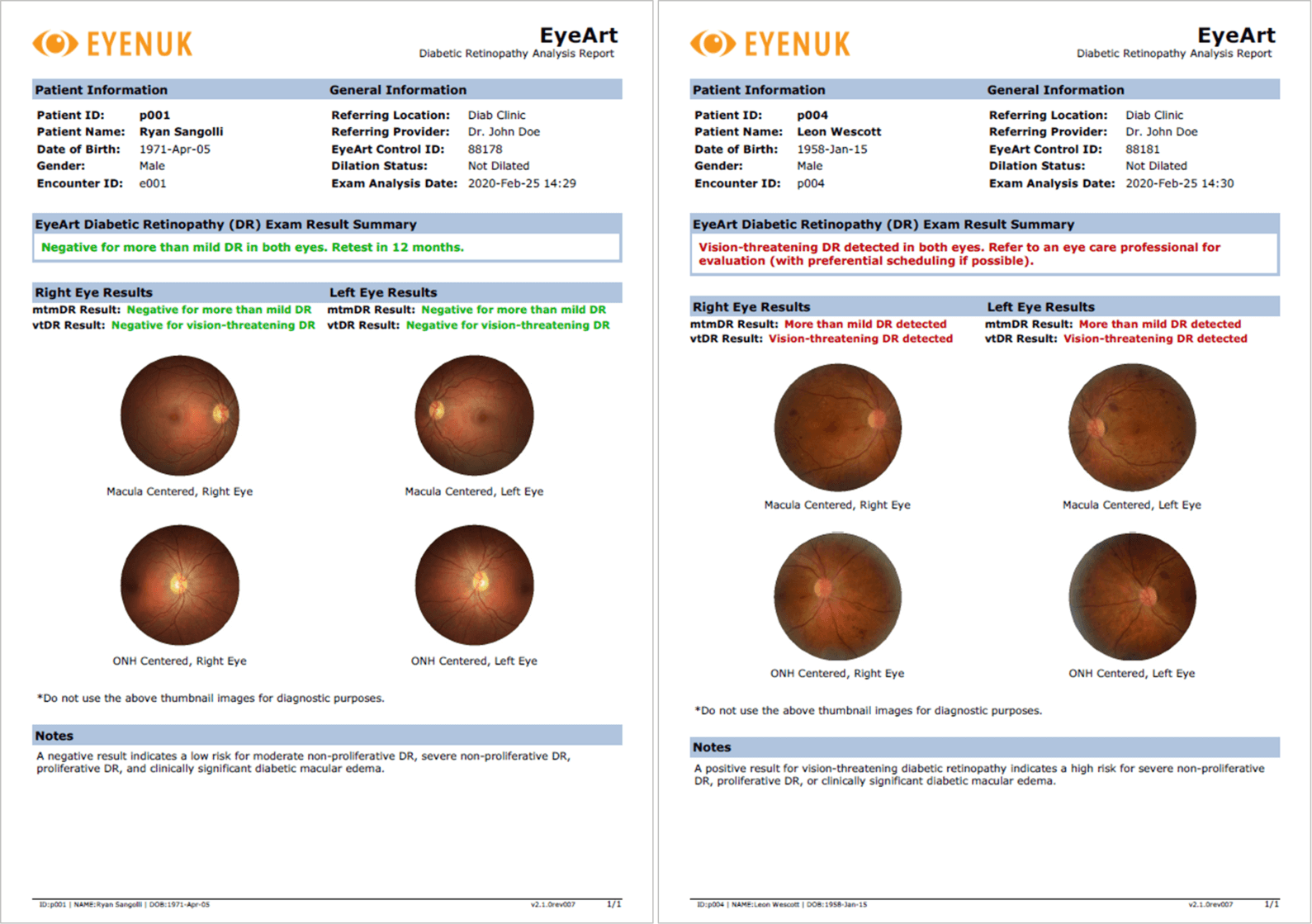
The EyeArt system autonomously analyzes patient’s retinal images to robustly detect signs of disease and returns an easy-to-read report in under 60 seconds. The report provides eye level and patient level diabetic retinopathy outputs and also indicates presence or absence of referable DR or vision threatening DR.
https://iovs.arvojournals.org/article.aspx?articleid=2638442
